Hydrogen production from water by solid oxide electrolysis
Solid oxide electrolysis cell (SOEC) is a high-temperature water electrolysis technology that uses YSZ and other materials as electrolytes to produce hydrogen through anode and cathode reactions. It has the advantages of low power consumption and high efficiency, and is suitable for waste heat recovery, but faces high cost and stability challenges.
Solid oxide electrolysis of water to produce hydrogen is a high-temperature water electrolysis technology. From the technical principle, SOEC can be divided into oxygen ion conduction SOEC and proton conduction SOEC.
Oxygen ion conduction SOEC working principle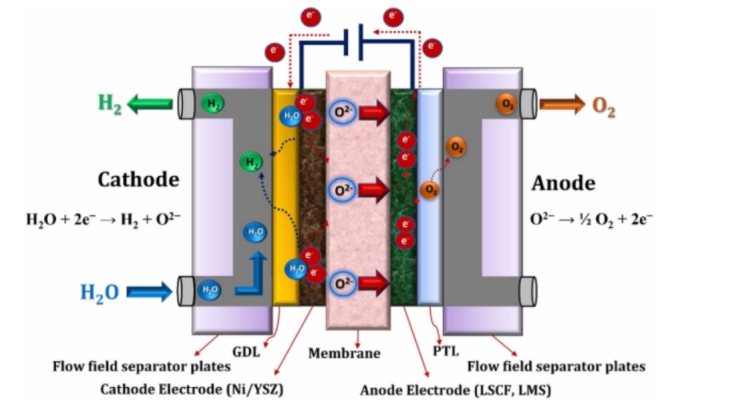
(Oxygen ion conduction SOEC working principle)
Working principle of proton-conduction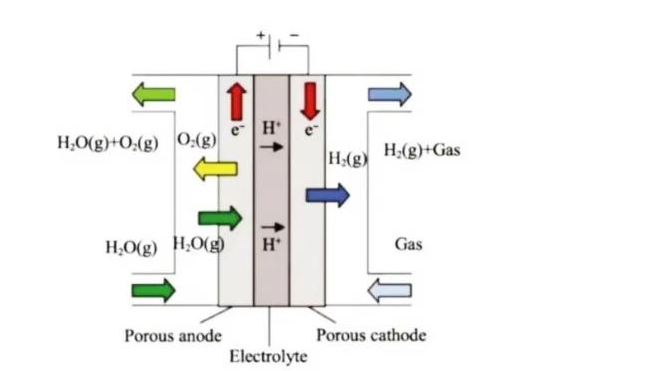
(Working principle of proton-conducting SOEC)
Oxygen ion conducting SOEC uses solid oxide as electrolyte, and the following chemical reactions occur at the anode and cathode respectively:
Anode: 2O²ˉ=O2+ 4e-
Cathode: 2H2O+4e-=2H2+2O²ˉ
The core components of SOEC include dense electrolyte and porous electrode, where the electrolyte is usually yttria stabilized zirconia (YSZ) material. At high temperatures of 600 to 1000°C, YSZ has excellent ionic conductivity and thermochemical stability, making it the preferred electrolyte material for SOEC.
In addition to YSZ, some other materials are also widely used in SOEC electrolytes. For example, scandia stabilized zirconia (ScSZ) and cerium oxide-based electrolytes, these materials also show good performance under certain conditions. In addition, lanthanum gallate-based electrolytes are gradually gaining attention, and the application of these materials provides a variety of choices for SOEC electrolytes.
In terms of electrode materials, hydrogen electrodes usually use Ni-YSZ metal ceramic composites, which not only have good conductivity, but also provide sufficient catalytic activity to promote hydrogen generation. Oxygen electrodes mostly use composites of strontium-doped lanthanum gallate (LSM) and YSZ, which can effectively catalyze oxygen generation and maintain stability at high temperatures.
The structure of SOEC is mainly divided into two types: tubular and flat. Tubular SOEC is the earliest type to be studied. Its main advantage is that it does not require additional sealing materials and the connection method is relatively simple. However, tubular SOEC also has disadvantages such as high cost and low power density. In contrast, flat SOEC has the advantages of high power density and low cost, so it has become a hot spot in current research. However, flat SOEC has great challenges in sealing, and it is necessary to overcome the stability of sealing materials under high temperature conditions.
The operating temperature of SOEC is usually as high as 600 to 1000℃, and the enthalpy of high-temperature water vapor is high, so the electrolysis voltage of SOEC can be as low as 1.3V, while the electrolysis voltage of alkaline electrolysis or proton exchange membrane (PEM) electrolysis is usually above 1.8V. Therefore, SOEC has obvious advantages in power consumption. Under the condition of minimum power consumption, 3kWh of electricity can produce 1 standard cubic meter of hydrogen. However, SOEC requires additional energy consumption to produce high-temperature water vapor, which has unique advantages in some special application scenarios, such as nuclear power hydrogen production.
Although SOEC has obvious advantages in power consumption and efficiency, its high operating temperature also brings some challenges and problems. The first is the cost issue. The cost of high-temperature materials and manufacturing processes is high. The second is the long start-up and shutdown time. Since SOEC needs to reach high temperature to operate, its startup and shutdown process is relatively slow. In addition, cycle life is also a key issue that needs to be solved. Under high-temperature operating conditions, the stability and durability of the material are facing challenges.
At present, the solid oxide water electrolysis hydrogen production technology is still in the demonstration and verification stage, and has not yet been realized in large-scale commercial applications. Despite the many challenges, SOEC technology has shown great potential in specific areas. For example, in the utilization of waste heat from nuclear power plants and high-temperature industrial waste heat recovery, SOEC technology can effectively convert these high-temperature heat sources into hydrogen, thereby achieving efficient utilization and conversion of energy.
In the future, with the continuous progress of materials science and manufacturing processes, SOEC technology is expected to overcome the current technical bottlenecks and achieve higher efficiency and lower costs. Further research and development will focus on improving the performance of electrolyte and electrode materials, extending the service life of equipment, and optimizing the overall design and operating parameters of the system. Through multi-faceted improvements and innovations, SOEC technology is expected to occupy an important position in the future hydrogen economy and become an important means of renewable energy utilization and hydrogen production.